Our Mission
VascuStim’s mission is to harness the power of precise bioelectric signaling for controlled protein expressions, including stem cell homing, to promote improved peripheral vascular circulation, healing, recovery and performance. When needed for the most severe cases we combine this with repeat deliveries of amniotic fluid membranes and our VS-15 multi-component stem cell + growth factor based organ regeneration and healing composition.
Our Story
VascuStim specializes in bioelectric signaling technologies that are redefining regeneration, healing, and recovery. The core of this technology platform are bioelectric signals for stem cell homing, proliferation and differentiation control as well as specific signals For severe cases we combine this with repeat applications of amniotic fluid membranes and repeat delivery of our VS-15 multi-component proprietary regeneration and healing composition comprised of stem cells, growth factors, selected exosomes, selected micro RNAs, selected alkaloids, nutrient hydrogel and matrix.
No organization has more experience in stem cell and bioelectric signaling based organ regenerative and recovery. Our electrical stimulator manufacturing partners in Southern California began building their first therapeutic electrical stimulation and ultrasonic therapy devices in 1955. In 1985 after consultations with Dr. Robert O. Becker the Author of The Body Electric we began our research program on improving blood circulation and organ regeneration. We published our first heart regeneration study in The Physiologist working with Dr. Race Kao in 1989 and our first bioelectric limb regeneration and ischemia treatment paper in CIRCULATION with Dr. Shinichi Kanno in 1999. We began filing a series of over 100 U.S.P.T.O. patent claims that year on bioelectric, stem cell and growth factor based organ regeneration and recovery. In the 1990’s we developed and patented the first endovascular percutaneous delivered aortic aneurysm and heart valve repair systems. In 2001 our Leonhardt team lead the completion of the historic landmark first ever non-surgical stem cell repair of a human heart. This led to publication of Pilot, Phase I, Phase II and Phase II/III study results. In 2016 we team with The Reems Lab at The University of Utah that had over a decade of experience in processing amniotic fluid membranes for healing applications. Over 400,000 patients have been treated to date with Leonhardt inventions for organ regeneration, treatment and recovery.


Team VascuStim
Chief Vascular Institute Advisor
Dr. Richard Neville
Chief Limb Ischemia Advisor
Dr. Jacob Cynamon
Chief Interventional Radiology Advisor
Dr. Harrison Lazarus
Chief Vascular Surgery Advisor
Dr. Juan Parodi
Chief Endovascular Advisor
Chief Medical Officer
Howard Leonhardt
Executive Chairman & CEO
Matt Fendrich
Vice President Physician Relations
Dr. Joanna Reems
Chief Advisor Amniotic Fluid Membranes
Dr. Jorge Genovese
Chief Bioelectric Regeneration Advisor
Dr. Nicolas Chronos
Chief Cardiology Advisor
Dr. Robert Kellar
Chief Wound Healing Advisor
Unmatched Experience
VascuStim is a unit of Leonhardt Ventures, led by Howard J. Leonhardt, which began organ regeneration research in the 1980’s. We completed with Dr. Race Kao and Dr. George Magovern our first stem cell repair of heart in a large animal in 1988 which was published in The Physiologist in 1989 Kao RL, Rizzo C, Magovern GJ: Satellite cells for myocardial regeneration. Physiologist 1989; 32: 220. In the 1990’s we gained world leadership positions in the development of cardiovascular balloon catheters, intravascular lungs, electro magnetic radiation and stem cell delivery catheters, aortic stent grafts and percutaneous heart valves. Our patented stent graft still holds the world’s leading share of endovascular aortic aneurysm repairs. In 1999 we published our first paper on bioelectric stimulation controlled limb ischemia treatment in the American Heart Association Journal CIRCULATION Circulation. 1999 May 25;99(20):2682-7. working with Dr. Shinichi Kanno. Our team went on that year to file the first in our series of over 100 U.S. patent claims for bioelectric stimulation controlled protein expressions – https://www.google.com/patents/US20050171578. In 2001 we made history by completing the first-in-man non-surgical stem cell repair of a human heart in The Netherlands working with Dr. Patrick Serruys, Dr. Pieter Smits, Dr. Doris Taylor and Dr. Warren Sherman – http://www.onlinejacc.org/content/accj/42/12/2063.full.pdf. In the 2000’s our team was the first to receive FDA permission to enter first stem cell therapy clinical trials for heart repair and later combination heart and gene therapy. Over 400,000 patients have been treated to date with Leonhardt inventions. Our vascular related inventions have been used by more than 2000 centers worldwide in over 40 countries. Recently we helped lead with collaborating associates limb salvage trials in Mexico and Czech Republic in 7 and 16 patients respectively with bioelectric stimulation and adipose derived cell compositions. We worked with a team in Denmark that completed a 47 patient clinical trial in Germany and Switzerland for wireless microcurrent treatment of non-healing ulcers. This data was published in the International Wound Journal in 2013 – http://onlinelibrary.wiley.com/doi/10.1111/iwj.12204/full. The Leonhardt team has helped lead clinical trials for stem cell therapies at over 38 leading research centers worldwide which includes 33 leading U.S. centers – https://www.clinicaltrials.gov/ct2/show/study/NCT00526253?term=MARVEL+bioheart&rank=1&show_locs=Y#locn.
Our Chief Medical Officer, Dr. Leslie Miller, former Chief of Cardiovascular Medicine at the University of Minnesota, has been involved in over a dozen stem cell therapy cardiovascular related clinical trials. He is co-editor of the textbook Stem Cell and Gene Therapies for Cardiovascular Disease https://www.elsevier.com/books/stem-cell-and-gene-therapy-for-cardiovascular-disease/perin/978-0-12-801888-0. Our Scientific Advisory Board is comprised of over 30 leading clinicians and scientists with unprecedented experience in the field – http://calxstars.com/scientific-advisory-board/.

The Reems Laboratory focuses on delivering novel cellular products that have the potential to improve the quality of life for patients. In her role as the Scientific Director for the Stem Cell Facility, Dr. Reems’ vision is to create a premier Cell Therapy and Regenerative Medicine Center of Excellence for the state of Utah that facilitates the translation of cutting-edge cell therapy and tissue engineered research into clinical products that extend and improve the quality of life of individuals suffering from debilitating diseases and injuries.
Dr. Reems obtained her B.S. in Medical Technology at the University of Colorado at Boulder. She then earned her Ph.D. in Biochemistry at the University of Colorado Health Sciences Center in Denver, Colorado. After her doctoral studies, Dr. Reems completed a Research Fellowship at Fred Hutchinson Cancer Research Center, with a focus on Regulators of Hematopoiesis. Prior to joining the University of Utah as faculty, Dr. Reems functioned as the Scientific Director of the Cell Therapy Facility at Puget Sound Blood Center in Seattle, Washington.

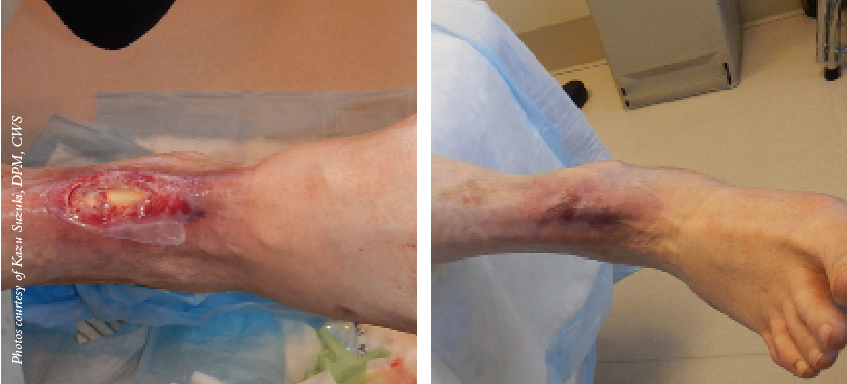
• Amniotic membrane has abundant stem cells that one can harvest without moral and ethical concerns.• Amniotic membrane lacks immunogenicity via a low expression or lack of expression of histocompatibility antigens.• Amniotic membrane has the ability to produce healing with little to no scarring. Along with the indirect contribution of amniotic membrane’s anti-inflammatory effects, there has been evidence of direct reduction of scarring. Tseng and colleagues demonstrated in a laboratory study that there was a direct anti-scarring action on ocular surface fibroblasts by suppressing transforming growth factor beta (TGF-b).3 The TGF-b is responsible for activation of fibroblasts and by down-regulating this process, there is a reduction and prevention of adhesion and fibrosis.• Amniotic membrane has the ability to impart anti-inflammatory effects by mediating pro-inflammatory cytokines such as interleukin (IL-6) and TNF-alpha. Tseng and colleagues noted that enzyme-linked immunosorbent assay (ELISA) extracts have high levels of IL-10, which counteracts inflammatory effects.3 In addition, they have found amniotic membrane down-regulates the expression and production of IL-1, and up-regulates interleukin-1 receptor antagonist (IL-1RA).
Given all these properties of amniotic membrane, this team seeks to initiate well designed studies to prove out the potential safety and efficacy of this product to heal these challenging diabetic wounds as well as treating critical limb ischemia, limb salvage and diabetic neuropathy.